Regeneration of Hair Cells
Authors: Rebecca Lewis, Jennifer Stone
To date, research shows that mammalian cochlear hair cells do not regenerate, either spontaneously or after damage. However, lower vertebrates (fishes, amphibians, reptiles, and birds) can spontaneously regrow hair cells, under normal conditions and/or after damage. Hair cell regeneration allows birds to hear again. These findings provide hope that, if hair cell regeneration were to be stimulated in mammals, the new hair cells would be sufficient to restore hearing function.
Regeneration in Birds
In their studies of birds, researchers confirmed that hair cells that are regenerated after damage in the auditory system can restore hearing. In other words, regenerated hair cells are effective in sending neural signals from the inner ear to the brain for processing and in this manner, they enable birds to hear with almost the exact same sensitivity as before damage.
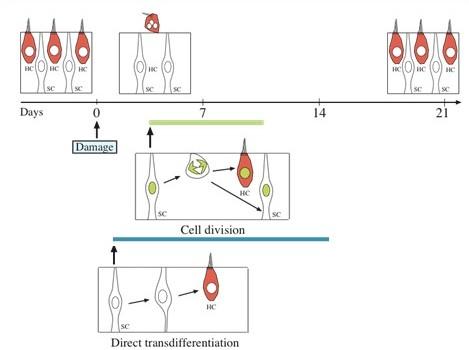
Cell division versus transdifferentiation
This regeneration can happen in one of two ways:
- (1) mitosis, during which supporting cells (SC, cells that surround hair cells) divide and form new cells, which become either a supporting cell or a hair cell (HC);
- (2) direct transdifferentiation, during which supporting cells (SC) change their phenotype and function to assume the identity of a hair cell (HC).
S. Blatrix, d’après B. Lewis
In order to understand how notch signaling works to increase levels of Atoh1, it is helpful to review the signaling pathway.
Here, you see three supporting cells in pink, and two hair cells in blue. After hair cells are damaged or die, expression of Delta becomes upregulated in some supporting cells. Supporting cells with elevated Delta inhibit surrounding supporting cells from becoming hair cells, because Delta binds to the notch receptor on those cells and inhibits Atoh1 expression. When Delta binds the notch receptor on an adjacent cell, the receptor is cleaved by gamma secretase, releasing the intracellular domain from the plasma membrane and allowing it to move to the nucleus. Once translocated to the nucleus, the NICD stimulates inhibitory transcription factors that block Atoh1 expression. Due to this process, called lateral inhibition, Atoh1 expression is blocked in some supporting cells while neighboring cells accumulate ATOH1 protein and differentiate into hair cells.
Regeneration in Mammals
Hope for regenerative therapies first emerged in the 1980’s when it was discovered that the inner ear of birds can regenerate hair cells that have been damaged by drugs or noise trauma and reconnect them to the brain. Many research groups around the world have since been trying to reproduce this regeneration in the mammalian cochlea. No luck so far: mammalian auditory hair cells cannot be regenerated!
Research continues in two directions.
One is to manage to ‘wake up’ some dormant stem cells that can be found in the adult cochlea.
The other (B) is to try to graft embryonic stem cells into the cochlea, then help them to differentiate into hair cells to take the place of those that are missing.
S. Blatrix, d’après J. Stone
In both of these cases, we would then need to help the new hair cells connect to the brain.
The challenge is great, and we cannot, at this stage, tell whether these methods will work, nor give a date of a potential therapy using this technique.
Restoration of Balance
When hair cells are lost in the non-mammalian vestibular system, they are readily regenerated. In birds the new hair cells are effective in restoring balance function. The vestibular organs are different from the auditory organs because their hair cells undergo continual turnover (death and regeneration), even in the absence of hair cell damage.
Several studies have shown that mature mammals (rodents) can also regenerate some of their vestibular hair cells in after hair cell damage. In newborn rodents, hair cells are readily replaced by supporting cell division and possibly by direct transdifferentiation. However, as rodents mature into adulthood, the number of supporting cells that divide is severely reduced. Only a subpopulation of hair cells is replaced, apparently by direct transdifferentiation.
The presence of some spontaneous hair cell regeneration in response to hair cell loss in mammals provides researchers with the opportunity to study mammalian hair cell regeneration and find experimental ways to boost it.