Cochlear fluids
Authors: Benjamin Delprat
Contributors: Sam Irving
The cochlear canals contain two types of fluid: perilymph and endolymph. Perilymph has a similar ionic composition as extracellular fluid found elsewhere in the body and fills the scalae tympani and vestibuli. Endolymph, found inside the cochlear duct (scala media), has a unique composition not found elsewhere in the body.
Composition of the cochlear fluids
A remarkable characteristic of the cochlea is the unique composition of endolymph. This liquid fills the scala media, and is very rich in potassium (150mM), very poor in sodium (1mM) and almost completely lacking in calcium (20-30 µM).
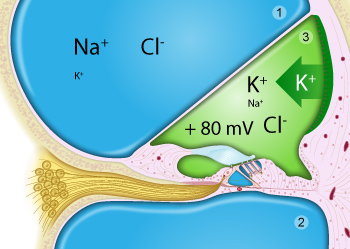
Perilymph (in blue) fills the scala vestibuli (1) and scala tympani (2).
Endolymph (in green) is limited to the scala media (= cochlear duct; 3), is very rich in potassium, secreted by the stria vascularis, and has a positive potential (+80mV) compared to perilymph.
Note that only the surface of the organ of Corti is bathed in endolymph (notably the stereocilia of the hair cells), whilst the main body of hair cells and support cells are bathed in perilymph.
Perilymph
There are two types of perilymph: the perilymph of the scala vestibuli, and that of the scala tympani. Both have a composition similar to cerebro-spinal fluid (CSF): rich in sodium (140mM) and poor in potassium (5mM) and calcium (1.2mM). The perilymph in the scala vestibuli comes from blood plasma across a hemto-perilymphatic barrier, whereas that of the scala tympani originates from CSF.
Endolymph
Endolymph is created from perilymph.
The endocochlear potential is the sum of two potentials: a positive potential caused by active secretion of K+ by the stria vascularis (120mV) and a negative potential created by the passive diffusion of K+ ions from the hair cells (40mV), which can be visualised after an anoxia.
Note that the ionic composition of endolymph develops prior to the endocochlear potential. In fact, in the mouse, endolymphatic ion concentrations are finalised during the first postnatal week. In comparison, the mouse endocochlear potential doesn’t develop until the second postnatal week and doesn’t reach its final value until the third week after birth.
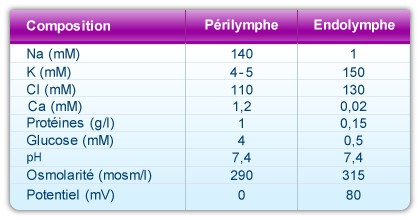
Composition and properties of the two cochlear fluids
Why endolymph?
It appears that the cochlea has found a way of regulating potassium flow without expending any energy (ATP). Generally speaking, if an ion enters a cell in a passive way, it requires an active mechanism to leave it, and vice versa.
Only the apical pole of the hair cells bathes in the potassium-rich endolymph, which has a positive potential of 80mV. K+ ions therefore enter these cells passively, as there is more potassium in the endolymph than in the hair cell and the latter have a resting potential of -60mV, which favours an influx of K+. These ions also leave the hair cells in a passive manner, due to the higher concentration of K+ ions inside the hair cell, compared to outside of the cell body bathed in perilymph. This all results in a significant saving of ATP by the hair cell.
Stria vascularis
The stria vascularis, a complex epithelial structure composed of various cell types, produces endolymph and releases it into the cochlea. The basal and marginal cells are true epithelial cells, whereas the intermediate cells are ‘melanocyte-like’. Intricate vasculature provides the oxygen and nutrients needed for the stria vascularis to function correctly.
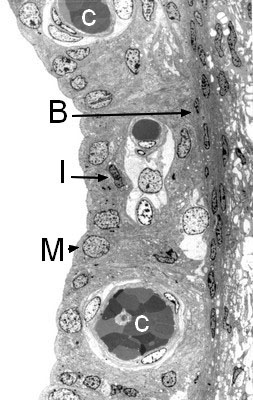
Structure of the stria vascularis (transmission electron microscopy)
Highly vascular (C), the stria vascularis is composed of three cell types:
- Marginal cells (M), which line the endolymphatic canal and have an essential role in ion exchange
- Intermediate cells (I), which are rich in the pigment melatonin
- Basal cells (B).
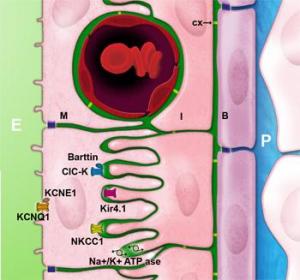
Potassium cycle
The potassium flow originating from the fibrocytes of the spiral ligament penetrates into the basal cells via a system of gap junctions composed of connexons, and hexamere 6 transmembrane proteins called connexins that form a hydrophilic canal of 2nm diameter. Connexins 26 and 30 (CX) are the most expressed connexins in the lateral wall of the cochlea and in the stria vascularis. Mutations in these two genes are the most common causes of prelingual human hearing loss.
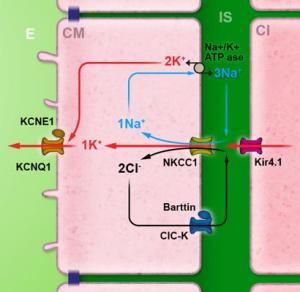
Potassium passes from basal cells (B) to intermediate cells (I) via the same network. It leaves the intermediate cells by the potassium channel Kir4.1 and rejoins the intrastrial space (in green). This passage of K+ ions across the intermediate cells’ membrane gives rise to the endocochlear potential. Mutations in Kir4.1 in humans are known to cause EAST syndrome (Epilepsy, Ataxia, Sensorineural deafness and Tubulopathy).
In order to generate a potential of 100mV, a weak concentration of potassium (around 1mM) is needed in the intrastrial space.
This task is carried out by Na, K-ATPase, which is expressed in the membrane of intermediate and marginal cells. Furthermore, the co-transporter NKCC1, expressed in the membrane of marginal cells, is also involved in this regulation by using the sodium gradient generated by the Na, K-ATPase to cause the ingression of potassium into the marginal cell.The influx mechanism of potassium ions into the marginal cells is very efficient, using a single ATP molecule to cause the influx of 5 molecules of potassium (2 using Na, K-APTase and 3 using the co-transporter NKCC1).
Finally, chlorine ions, which enter the cell at the same time as sodium and potassium ions via the co-transporter NKCC1, are expelled by CIC-K chlorine channels associated with their regulating beta-subunit, barttine. Mutations in barttine expression are responsible for Bartter’s syndrome; causing both tubulopathy and hearing loss. Potassium is finally secreted into scala media via KCNQ1 potassium channels associated to their regulating subunit KCNE1. Mutations in these two genes give rise to Jervell and Lange-Nielsen syndrome, causing bilateral hearing loss and a long cardiac QT.